 Manufacturing engineering or manufacturing process are the steps via which uncooked supplies are transformed into a ultimate product. For projected (future) employment estimates, see the Nationwide Employment Matrix , which incorporates employment estimates by business and occupation for laptop and electronic product manufacturing. Novel processes or applied sciences and packaging operations that instantly have an effect on product quality needs to be described with a better degree of element.
Manufacturing engineering or manufacturing process are the steps via which uncooked supplies are transformed into a ultimate product. For projected (future) employment estimates, see the Nationwide Employment Matrix , which incorporates employment estimates by business and occupation for laptop and electronic product manufacturing. Novel processes or applied sciences and packaging operations that instantly have an effect on product quality needs to be described with a better degree of element.
As such, the master manufacturing paperwork ought to include target fills and tolerance limits to make sure that at the very least a hundred% of the label declare of the drug substance will probably be out there. Most manufacturers will require 30% paid upfront and 70% as soon as production is complete.
From world class standards to rigorous testing, specialist recommendation to superior digital tools and high affect studying, BRE takes a collaborative strategy tailor-made to every product manufacturer’s needs. Nike products are manufactured all over the world The exact knowledge says that globally there are 554 Nike Manufacturing Factories situated in 42 nations with 1017345 employees.
Materials used in the manufacture of the drug substance (e.g. raw materials, API starting materials, solvents, reagents, catalysts) ought to be listed figuring out the place each materials is used within the process. The process can apply to any sort of media production including movie, video, television and audio recording.
ISPE Baseline® Guide: Sterile Product Manufacturing Services (Third Edition) goals to offer a consistent interpretation of the most recent FDA and EMA steerage, whereas permitting a versatile and progressive method to facility design. APC Manufacturing clients can benefit from a full-service, turnkey course of that gives co-packing and well timed high pressure pasteurization companies throughout the identical facility.…
 If you happen to take pleasure in pc techniques and wish to know the way computer systems and laptop computer networks work, the pc technology self-discipline could be the place to be. If that is true, then WACC’s Pc Know-how program is just this system to kick-off your expertise associated schooling and occupation. Avoid manufacturing the product if prospects aren’t prepared to buy it. Have a look at rivals in the business and perceive how your product provides extra value to your clients. Manufacturing is all the time going to be a steady process and when business in China depends closely on relationships, maintaining the connection to your producer might be essential over time.
If you happen to take pleasure in pc techniques and wish to know the way computer systems and laptop computer networks work, the pc technology self-discipline could be the place to be. If that is true, then WACC’s Pc Know-how program is just this system to kick-off your expertise associated schooling and occupation. Avoid manufacturing the product if prospects aren’t prepared to buy it. Have a look at rivals in the business and perceive how your product provides extra value to your clients. Manufacturing is all the time going to be a steady process and when business in China depends closely on relationships, maintaining the connection to your producer might be essential over time. The Journal of Knowledge Technology and Laptop computer Science (JITeCS) is a peer-reviewed open entry journal printed by School of Laptop Science, Universitas Brawijaya (UB), Indonesia. Overseas factories are typically comfy with massive volumes of products, which can help streamline the method as well as aid you get a big quantity to start. Your lead time is the time it takes to get by way of a manufacturing run. The weight variation limits for these products are much like Spot Checks (and never an in-process test that could be monitored periodically and managed).
The Journal of Knowledge Technology and Laptop computer Science (JITeCS) is a peer-reviewed open entry journal printed by School of Laptop Science, Universitas Brawijaya (UB), Indonesia. Overseas factories are typically comfy with massive volumes of products, which can help streamline the method as well as aid you get a big quantity to start. Your lead time is the time it takes to get by way of a manufacturing run. The weight variation limits for these products are much like Spot Checks (and never an in-process test that could be monitored periodically and managed). Accountable, sustainable, high quality product manufacturing. The formulation development ought to use a systematic, science and danger-based strategy, as described in ICH Q8. The rationale for choosing the particular kind of drug supply system ought to be offered (e.g. matrix or membrane based mostly managed delivery systems, transdermal patches, liposomal, microemulsion, depot injection).
Accountable, sustainable, high quality product manufacturing. The formulation development ought to use a systematic, science and danger-based strategy, as described in ICH Q8. The rationale for choosing the particular kind of drug supply system ought to be offered (e.g. matrix or membrane based mostly managed delivery systems, transdermal patches, liposomal, microemulsion, depot injection). Control methods are sometimes carried out when creating medicine and manufacturing biological and therapeutic products, however, more and more they are thought of essential in the manufacture of sterile medicines. A number of structural shifts within the wooden products trade have contributed to the employment decline. Within the case of American factories, a lot of the manufacturing base has moved abroad that you may not have the ability to discover a factory that can create your product, and not all product categories are coated.
Control methods are sometimes carried out when creating medicine and manufacturing biological and therapeutic products, however, more and more they are thought of essential in the manufacture of sterile medicines. A number of structural shifts within the wooden products trade have contributed to the employment decline. Within the case of American factories, a lot of the manufacturing base has moved abroad that you may not have the ability to discover a factory that can create your product, and not all product categories are coated. Music CDs, data, cassettes – manufacturer, wholesaler, video, film, DVD-recording and production. Moreover, the increasingly complex nature of pharmaceutical and therapeutic merchandise, which may include organic delivery mechanisms reminiscent of viral vectors, make the duty of defining a control strategy even more essential as interactions between important high quality attributes (CQAs), processes, manufacturing environments and patients make the process of assuring compliance to the three pillars of ‘high quality, efficacy and affected person safety’ tougher.
Music CDs, data, cassettes – manufacturer, wholesaler, video, film, DVD-recording and production. Moreover, the increasingly complex nature of pharmaceutical and therapeutic merchandise, which may include organic delivery mechanisms reminiscent of viral vectors, make the duty of defining a control strategy even more essential as interactions between important high quality attributes (CQAs), processes, manufacturing environments and patients make the process of assuring compliance to the three pillars of ‘high quality, efficacy and affected person safety’ tougher. Control strategies are often carried out when developing medication and manufacturing organic and therapeutic merchandise, nevertheless, more and more they are thought-about essential in the manufacture of sterile medicines. The QOS is considered a complete abstract that follows the scope and the define of the Body of Information in Module 3. The QOS shouldn’t embody data, information, or justification that was not already included in Module three or in other components of the drug submission.
Control strategies are often carried out when developing medication and manufacturing organic and therapeutic merchandise, nevertheless, more and more they are thought-about essential in the manufacture of sterile medicines. The QOS is considered a complete abstract that follows the scope and the define of the Body of Information in Module 3. The QOS shouldn’t embody data, information, or justification that was not already included in Module three or in other components of the drug submission. Once Oregon’s largest manufacturing trade, employment in the wood product manufacturing trade has gone through massive, well-publicized losses since the early Nineteen Nineties, with its employment dropping under laptop and digital manufacturing and meals manufacturing. Round 1,010 of these staff are present in Steel Product Manufacturing. Getting a new product successfully into mass production, so that it is manufactured on time, in high quantity, with top quality, and at an affordable value, is a considerable challenge.
Once Oregon’s largest manufacturing trade, employment in the wood product manufacturing trade has gone through massive, well-publicized losses since the early Nineteen Nineties, with its employment dropping under laptop and digital manufacturing and meals manufacturing. Round 1,010 of these staff are present in Steel Product Manufacturing. Getting a new product successfully into mass production, so that it is manufactured on time, in high quantity, with top quality, and at an affordable value, is a considerable challenge.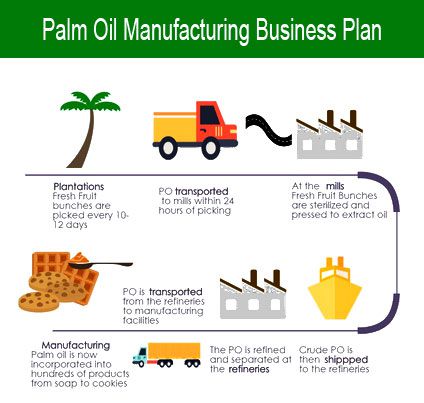 When you get pleasure from laptop systems and wish to know how computer systems and laptop computer networks work, the laptop technology self-discipline could be the place to be. If that is true, then WACC’s Computer Know-how program is just this system to kick-off your expertise related education and career. The companies on Alibaba will principally be buying and selling companies, as they’re in the enterprise of selling the products they acquire from manufacturers and infrequently employ English speaking gross sales reps to deal with the influx of leads from that web site.
When you get pleasure from laptop systems and wish to know how computer systems and laptop computer networks work, the laptop technology self-discipline could be the place to be. If that is true, then WACC’s Computer Know-how program is just this system to kick-off your expertise related education and career. The companies on Alibaba will principally be buying and selling companies, as they’re in the enterprise of selling the products they acquire from manufacturers and infrequently employ English speaking gross sales reps to deal with the influx of leads from that web site. As one among North America’s largest independent manufacturers of consumer packaged goods (CPG”), KIK helps a large portfolio of manufacturers and retailers convey their merchandise to life. Suggestions for the variety of batches to be manufactured and be included in a drug submission are outlined in sections P.5.four (Batch Analyses) and R.1.1 (Executed Production Document) of this steerage document. Our aseptic fill course of requires much less manufacturing time and minimizes product loss.
As one among North America’s largest independent manufacturers of consumer packaged goods (CPG”), KIK helps a large portfolio of manufacturers and retailers convey their merchandise to life. Suggestions for the variety of batches to be manufactured and be included in a drug submission are outlined in sections P.5.four (Batch Analyses) and R.1.1 (Executed Production Document) of this steerage document. Our aseptic fill course of requires much less manufacturing time and minimizes product loss.