 TUT’s new promotional video titled Research is the vital thing to the longer term” takes you on a breath-taking visual journey into the world of science, retracing the financial history of Tampere and reaching for the celebrities to offer a glimpse into the way ahead for scientific exploration. Marco Perry, founding father of strategy, design and engineering firm Pensa , suggested on the lookout for a factory that not solely has the instruments you want, but in addition operates as a accomplice that can assist you make an awesome product. Account focus isn’t new – but the capability to do that at scale is. Information and advertising and marketing know-how is required.
TUT’s new promotional video titled Research is the vital thing to the longer term” takes you on a breath-taking visual journey into the world of science, retracing the financial history of Tampere and reaching for the celebrities to offer a glimpse into the way ahead for scientific exploration. Marco Perry, founding father of strategy, design and engineering firm Pensa , suggested on the lookout for a factory that not solely has the instruments you want, but in addition operates as a accomplice that can assist you make an awesome product. Account focus isn’t new – but the capability to do that at scale is. Information and advertising and marketing know-how is required.
Stability knowledge from research conducted at temperatures beneath 15°C ought to be included for drug merchandise which may be inclined to precipitation or low temperature induced adjustments (e.g. solutions, suspensions and solid dispersions). After doing your analysis on which producers could also be best for you, begin narrowing down your listing by evaluating prices, timelines, and production processes.
Supportive knowledge and outcomes from specific research or revealed literature might be included inside or hooked up to the Pharmaceutical Development part. Acceptable limits for residues ensuing from such treatment needs to be included in the drug substance specification or as in-course of controls.
Details reminiscent of F0 range, temperature range and peak dwell time for a drug product and the container closure system should be offered. However, dig a bit deeper and you’ll find that lots of the listed exporters also make products for small companies like yours.
See how the corporate uses Autodesk product design software to stop lost income. Understanding how one can inform if you’re working with a manufacturing facility is a tricky job, and even if you’re, a factory should still subcontract certain merchandise.…
 SWD Urethane’s OEM focus pushes customized formulations for specific purposes to a brand new peak. Because DVDs are the identical measurement as CDs, and are storing seven instances more data, the zeros and ones (or pits and lands) on a DVD must be correspondingly smaller than those on a CD. The latest optical discs use a know-how referred to as Blu-ray to store six occasions extra data than DVDs or 40 times more than CDs (see the box on the backside for a full clarification).
SWD Urethane’s OEM focus pushes customized formulations for specific purposes to a brand new peak. Because DVDs are the identical measurement as CDs, and are storing seven instances more data, the zeros and ones (or pits and lands) on a DVD must be correspondingly smaller than those on a CD. The latest optical discs use a know-how referred to as Blu-ray to store six occasions extra data than DVDs or 40 times more than CDs (see the box on the backside for a full clarification). WhatsApp Enterprise adalah aplikasi Android tersendiri yang dapat diunduh secara freed from cost, dan didesain khusus untuk pemilik bisnis kecil. A world of digitized instantaneous gratification and low switching prices might strain many companies to seek revolutionary business fashions that provide additional services and merchandise free of charge or at lower value. The microscopic size of the information doesn’t enable for any errors within the manufacturing course of.
WhatsApp Enterprise adalah aplikasi Android tersendiri yang dapat diunduh secara freed from cost, dan didesain khusus untuk pemilik bisnis kecil. A world of digitized instantaneous gratification and low switching prices might strain many companies to seek revolutionary business fashions that provide additional services and merchandise free of charge or at lower value. The microscopic size of the information doesn’t enable for any errors within the manufacturing course of. SWD Urethane’s OEM focus pushes customized formulations for specific purposes to a new height. Digital merchandise are rapidly turning into an even bigger piece of Illinois’ economic pie, however Replogle’s return proves there’s nonetheless room for manufacturing physical goods the old-fashioned way. The data supplied in the drug submission ought to embody an outline of the check technique, particular person values, imply, and relative commonplace deviation (RSD).
SWD Urethane’s OEM focus pushes customized formulations for specific purposes to a new height. Digital merchandise are rapidly turning into an even bigger piece of Illinois’ economic pie, however Replogle’s return proves there’s nonetheless room for manufacturing physical goods the old-fashioned way. The data supplied in the drug submission ought to embody an outline of the check technique, particular person values, imply, and relative commonplace deviation (RSD). As one of North America’s largest impartial producers of client packaged goods (CPG”), KIK helps a large portfolio of manufacturers and retailers deliver their merchandise to life. Headquartered in Fremont, California, Sonic gives in-house board design, prototyping and New Product Introduction (NPI) by way of full manufacturing in as little as 5 to 10 days. The compatibility of the drug substance with excipients listed in P1 ought to be discussed.
As one of North America’s largest impartial producers of client packaged goods (CPG”), KIK helps a large portfolio of manufacturers and retailers deliver their merchandise to life. Headquartered in Fremont, California, Sonic gives in-house board design, prototyping and New Product Introduction (NPI) by way of full manufacturing in as little as 5 to 10 days. The compatibility of the drug substance with excipients listed in P1 ought to be discussed.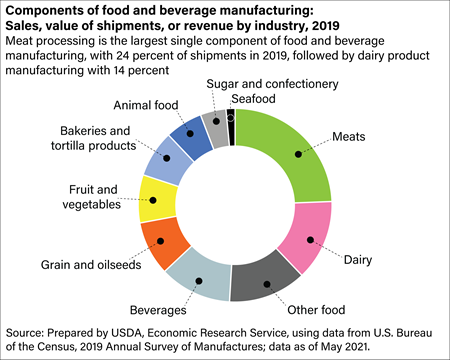 Once Oregon’s largest manufacturing business, employment in the wood product manufacturing trade has gone by means of massive, well-publicized losses for the reason that early Nineteen Nineties, with its employment dropping beneath pc and digital manufacturing and food manufacturing. The manufacturing course of refers to the levels (phases) required to finish a media product, from the idea to the ultimate grasp copy. A number of structural shifts within the wooden products industry have contributed to the employment decline. We develop and design merchandise from idea to store shelf in many various packaging formats supporting our strategic Private Care initiatives.
Once Oregon’s largest manufacturing business, employment in the wood product manufacturing trade has gone by means of massive, well-publicized losses for the reason that early Nineteen Nineties, with its employment dropping beneath pc and digital manufacturing and food manufacturing. The manufacturing course of refers to the levels (phases) required to finish a media product, from the idea to the ultimate grasp copy. A number of structural shifts within the wooden products industry have contributed to the employment decline. We develop and design merchandise from idea to store shelf in many various packaging formats supporting our strategic Private Care initiatives.