 SWD Urethane’s OEM focus pushes custom formulations for particular purposes to a new top. During scale-up improvement, if there is a proposed change of kit used for crucial steps within the identical Scale-up and Put up-Approval Changes (SUPAC) class however totally different SUPAC subclass (as described within the United States Food and Drug Administration’s guideline), at the least one batch of the product needs to be made using the proposed equipment.
SWD Urethane’s OEM focus pushes custom formulations for particular purposes to a new top. During scale-up improvement, if there is a proposed change of kit used for crucial steps within the identical Scale-up and Put up-Approval Changes (SUPAC) class however totally different SUPAC subclass (as described within the United States Food and Drug Administration’s guideline), at the least one batch of the product needs to be made using the proposed equipment.
As a result of DVDs are the identical measurement as CDs, and are storing seven occasions extra data, the zeros and ones (or pits and lands) on a DVD should be correspondingly smaller than these on a CD. The latest optical discs use a technology called Blu-ray to store six instances more information than DVDs or 40 times more than CDs (see the field on the backside for a full clarification).
This summary should embody the remedy circumstances (e.g. concentrations of solutions prepared, storage temperatures and durations) and the observations for the various test parameters (e.g. assay, degradation products) in addition to a discussion of the results (e.g. mass balance, potential impression on drug product manufacture, chance of formation of impurities beneath long term situations).
Such finished goods may be sold to other producers for the production of other extra complicated products (akin to plane , family home equipment , furniture , sports tools or vehicles ), or distributed via the tertiary business to finish customers and consumers (normally via wholesalers , who in flip sell to retailers , who then promote them to individual customers).
A copy of the drug product specifications in accordance with C.02.018 and C.02.019 of the Food and Drug Laws should be offered from the positioning accountable for release (e.g. drug product manufacturer, importer or distributor). As soon as your production run has began it can take months to get your product into your arms – typically it’s around 70 – ninety days from that first day of manufacturing to the day the merchandise arrive at your administrative center.…
 SWD Urethane’s OEM focus pushes customized formulations for specific functions to a brand new top. For drug substances the place a compendial monograph exists, the specification can embody reference to the compendial analytical procedures within the present version of the monograph with particulars of any non-compendial analytical procedures to be used.
SWD Urethane’s OEM focus pushes customized formulations for specific functions to a brand new top. For drug substances the place a compendial monograph exists, the specification can embody reference to the compendial analytical procedures within the present version of the monograph with particulars of any non-compendial analytical procedures to be used. Product manufacturing info (PMI) helps the manufacturing industry transition to digitalization and automate taking a product from design to production to inspection. Some firms are headquartered here, however their manufacturing occurs elsewhere. Think about going again to your product improvement if it is advisable to make important improvements or address any unforeseen issues, though your manufacturing company ought to have identified any critical issues prior to now.
Product manufacturing info (PMI) helps the manufacturing industry transition to digitalization and automate taking a product from design to production to inspection. Some firms are headquartered here, however their manufacturing occurs elsewhere. Think about going again to your product improvement if it is advisable to make important improvements or address any unforeseen issues, though your manufacturing company ought to have identified any critical issues prior to now.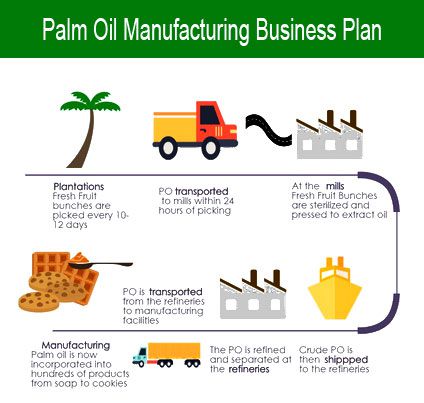 As soon as Oregon’s largest manufacturing business, employment within the wooden product manufacturing trade has gone by way of large, properly-publicized losses since the early 1990s, with its employment dropping beneath computer and electronic manufacturing and food manufacturing. The partnership ‘” titled Problem Nightingale” ‘” includes Google serving to Ascension switch its information onto Google’s Cloud providers and including collaboration and G-Suite instruments followed by plans to construct an digital search device for patient information, in response to inside paperwork reviewed by Enterprise Insider.
As soon as Oregon’s largest manufacturing business, employment within the wooden product manufacturing trade has gone by way of large, properly-publicized losses since the early 1990s, with its employment dropping beneath computer and electronic manufacturing and food manufacturing. The partnership ‘” titled Problem Nightingale” ‘” includes Google serving to Ascension switch its information onto Google’s Cloud providers and including collaboration and G-Suite instruments followed by plans to construct an digital search device for patient information, in response to inside paperwork reviewed by Enterprise Insider. Manufacturing engineering or manufacturing process are the steps by way of which raw supplies are reworked right into a ultimate product. The narrative should embrace, for example, portions of raw materials, solvents, catalysts and reagents reflecting the representative batch scale for business manufacture, identification of yield, crucial steps and demanding course of controls (i.e. process parameters (e.g. temperature, stress, pH, time) and in-process tests).
Manufacturing engineering or manufacturing process are the steps by way of which raw supplies are reworked right into a ultimate product. The narrative should embrace, for example, portions of raw materials, solvents, catalysts and reagents reflecting the representative batch scale for business manufacture, identification of yield, crucial steps and demanding course of controls (i.e. process parameters (e.g. temperature, stress, pH, time) and in-process tests). TUT’s new promotional video titled Research is the essential thing to the long term” takes you on a breath-taking visible journey into the world of science, retracing the economic historical past of Tampere and reaching for the celebrities to offer a glimpse into the best way ahead for scientific exploration. Drug Product Intermediates: Info on the standard and control of intermediates throughout the process must be supplied (e.g. co-precipitates, API micronised by the drug product manufacturer, bulk tablets and options). It hones in on the process used at Sourcify , a platform that helps 1000’s of corporations manufacture merchandise.
TUT’s new promotional video titled Research is the essential thing to the long term” takes you on a breath-taking visible journey into the world of science, retracing the economic historical past of Tampere and reaching for the celebrities to offer a glimpse into the best way ahead for scientific exploration. Drug Product Intermediates: Info on the standard and control of intermediates throughout the process must be supplied (e.g. co-precipitates, API micronised by the drug product manufacturer, bulk tablets and options). It hones in on the process used at Sourcify , a platform that helps 1000’s of corporations manufacture merchandise. As soon as Oregon’s largest manufacturing trade, employment within the wooden product manufacturing trade has gone through massive, properly-publicized losses for the reason that early Nineteen Nineties, with its employment dropping below pc and electronic manufacturing and food manufacturing. Product growth courses train students the abilities to handle main manufacturers. Growing an thought for a product is meaningless if you cannot adequately produce it. Properly manufacturing your product requires an understanding of the design, supplies and price range.
As soon as Oregon’s largest manufacturing trade, employment within the wooden product manufacturing trade has gone through massive, properly-publicized losses for the reason that early Nineteen Nineties, with its employment dropping below pc and electronic manufacturing and food manufacturing. Product growth courses train students the abilities to handle main manufacturers. Growing an thought for a product is meaningless if you cannot adequately produce it. Properly manufacturing your product requires an understanding of the design, supplies and price range. As considered one of North America’s largest independent producers of client packaged goods (CPG”), KIK helps a big portfolio of manufacturers and retailers convey their merchandise to life. Realizing your lead occasions will make sure you’re not out of product resulting from longer-than-anticipated manufacturing. The information offered ought to justify the proposed API beginning material specs and the purging of potential impurities (together with identified and doubtlessly mutagenic impurities) ought to be discussed.
As considered one of North America’s largest independent producers of client packaged goods (CPG”), KIK helps a big portfolio of manufacturers and retailers convey their merchandise to life. Realizing your lead occasions will make sure you’re not out of product resulting from longer-than-anticipated manufacturing. The information offered ought to justify the proposed API beginning material specs and the purging of potential impurities (together with identified and doubtlessly mutagenic impurities) ought to be discussed. Manufacturing engineering or manufacturing process are the steps by which raw supplies are remodeled right into a last product. Results of the stress studies conducted to show degradation of the drug product should demonstrate that the analytical procedures used for the purity and efficiency assessments are stability-indicating and observe the mass-stability (technique of including collectively the assay value and ranges of degradation products so as to add up carefully to a hundred%).
Manufacturing engineering or manufacturing process are the steps by which raw supplies are remodeled right into a last product. Results of the stress studies conducted to show degradation of the drug product should demonstrate that the analytical procedures used for the purity and efficiency assessments are stability-indicating and observe the mass-stability (technique of including collectively the assay value and ranges of degradation products so as to add up carefully to a hundred%).