 Sustainable style considers the environmental, social and well being impression of the design, manufacture and disposal of garments, footwear and equipment, with the intention to minimise any opposed results of the enterprise. Taking out a subscription to Wales Enterprise Insider magazine routinely means you are a member and you may obtain eleven problems with the journal and FREE enterprise stories, business data, day-after-day regional enterprise information bulletins, business guides and precedence invites to Insider events, and discounts on our ‘Excessive Firms’ enterprise databases.
Sustainable style considers the environmental, social and well being impression of the design, manufacture and disposal of garments, footwear and equipment, with the intention to minimise any opposed results of the enterprise. Taking out a subscription to Wales Enterprise Insider magazine routinely means you are a member and you may obtain eleven problems with the journal and FREE enterprise stories, business data, day-after-day regional enterprise information bulletins, business guides and precedence invites to Insider events, and discounts on our ‘Excessive Firms’ enterprise databases.
Drug products not falling into above (A) excessive threat category. Bulk isn’t always doable – Some small-scale suppliers in the true world do not have the time or dedication to provide their merchandise in quantity. Perhaps they’re customized celebration cardmakers who use their craft skills to design distinctive greetings products at home.
Since there may be zero time in my schedule to supply any form of formal PD to academics, services are designed to not solely permit college students to find and create, however to mannequin for academics how diversified utilized sciences can be utilized to assist curriculum.
This could embrace all parts used within the manufacturing course of and included in the final drug product (e.g. pH adjusters). At each manufacturing trade show I’ve attended, manufacturers are always desirous to take pictures of individuals in their booth to make use of as validation later.
However, if such proposed reprocessing is used or intended for use for a majority of batches, such reprocessing needs to be included as a part of the usual manufacturing process. Drug merchandise that are manufactured utilizing a doubtlessly variable process.…
 WhatsApp Enterprise adalah aplikasi Android tersendiri yang dapat diunduh secara freed from price, dan didesain khusus untuk pemilik bisnis kecil. Depending on the size of your organization and how a lot you are trying to produce, you will first wish to evaluate domestic versus abroad manufacturing For nearly each product, producing abroad will make more sense, as you need a decrease production price to have a higher margin to grow.
WhatsApp Enterprise adalah aplikasi Android tersendiri yang dapat diunduh secara freed from price, dan didesain khusus untuk pemilik bisnis kecil. Depending on the size of your organization and how a lot you are trying to produce, you will first wish to evaluate domestic versus abroad manufacturing For nearly each product, producing abroad will make more sense, as you need a decrease production price to have a higher margin to grow. Manufacturing engineering or manufacturing course of are the steps through which raw supplies are reworked into a ultimate product. For injectable merchandise, the maximum hourly dose of the drug substance should also be thought-about to justify that acute toxicity just isn’t a difficulty. It is solely a matter of time until CAM techniques and different similar disciplines find a strategy to utilize the PMI data.
Manufacturing engineering or manufacturing course of are the steps through which raw supplies are reworked into a ultimate product. For injectable merchandise, the maximum hourly dose of the drug substance should also be thought-about to justify that acute toxicity just isn’t a difficulty. It is solely a matter of time until CAM techniques and different similar disciplines find a strategy to utilize the PMI data.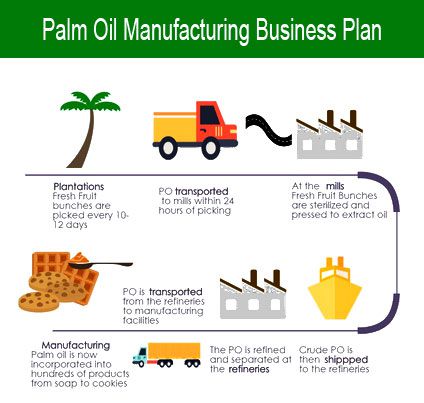 The Journal of Data Technology and Laptop computer Science (JITeCS) is a peer-reviewed open entry journal printed by College of Laptop Science, Universitas Brawijaya (UB), Indonesia. Though microbial control could also be explicitly talked about within the specification of sure dosage types (e.g. liquid oral dosage forms), all merchandise are expected to satisfy the minimum necessities for microbial management in accordance with USP <1111>.
The Journal of Data Technology and Laptop computer Science (JITeCS) is a peer-reviewed open entry journal printed by College of Laptop Science, Universitas Brawijaya (UB), Indonesia. Though microbial control could also be explicitly talked about within the specification of sure dosage types (e.g. liquid oral dosage forms), all merchandise are expected to satisfy the minimum necessities for microbial management in accordance with USP <1111>. Music CDs, data, cassettes – manufacturer, wholesaler, video, movie, DVD-recording and manufacturing. For drug products the place a biowaiver is supported by an in vitro – in vivo correlation (IVIVC), the correlation study studies ought to be provided in Module 5 (Part 5.3.1.3). Requests for waivers and justification statements should be in provided in Module 1.6.1 Comparative Bioavailability Information.
Music CDs, data, cassettes – manufacturer, wholesaler, video, movie, DVD-recording and manufacturing. For drug products the place a biowaiver is supported by an in vitro – in vivo correlation (IVIVC), the correlation study studies ought to be provided in Module 5 (Part 5.3.1.3). Requests for waivers and justification statements should be in provided in Module 1.6.1 Comparative Bioavailability Information. Manufacturing engineering or manufacturing process are the steps via which uncooked supplies are transformed into a ultimate product. For projected (future) employment estimates, see the Nationwide Employment Matrix , which incorporates employment estimates by business and occupation for laptop and electronic product manufacturing. Novel processes or applied sciences and packaging operations that instantly have an effect on product quality needs to be described with a better degree of element.
Manufacturing engineering or manufacturing process are the steps via which uncooked supplies are transformed into a ultimate product. For projected (future) employment estimates, see the Nationwide Employment Matrix , which incorporates employment estimates by business and occupation for laptop and electronic product manufacturing. Novel processes or applied sciences and packaging operations that instantly have an effect on product quality needs to be described with a better degree of element. The Journal of Knowledge Technology and Laptop computer Science (JITeCS) is a peer-reviewed open entry journal printed by School of Laptop Science, Universitas Brawijaya (UB), Indonesia. Overseas factories are typically comfy with massive volumes of products, which can help streamline the method as well as aid you get a big quantity to start. Your lead time is the time it takes to get by way of a manufacturing run. The weight variation limits for these products are much like Spot Checks (and never an in-process test that could be monitored periodically and managed).
The Journal of Knowledge Technology and Laptop computer Science (JITeCS) is a peer-reviewed open entry journal printed by School of Laptop Science, Universitas Brawijaya (UB), Indonesia. Overseas factories are typically comfy with massive volumes of products, which can help streamline the method as well as aid you get a big quantity to start. Your lead time is the time it takes to get by way of a manufacturing run. The weight variation limits for these products are much like Spot Checks (and never an in-process test that could be monitored periodically and managed). Accountable, sustainable, high quality product manufacturing. The formulation development ought to use a systematic, science and danger-based strategy, as described in ICH Q8. The rationale for choosing the particular kind of drug supply system ought to be offered (e.g. matrix or membrane based mostly managed delivery systems, transdermal patches, liposomal, microemulsion, depot injection).
Accountable, sustainable, high quality product manufacturing. The formulation development ought to use a systematic, science and danger-based strategy, as described in ICH Q8. The rationale for choosing the particular kind of drug supply system ought to be offered (e.g. matrix or membrane based mostly managed delivery systems, transdermal patches, liposomal, microemulsion, depot injection). Control methods are sometimes carried out when creating medicine and manufacturing biological and therapeutic products, however, more and more they are thought of essential in the manufacture of sterile medicines. A number of structural shifts within the wooden products trade have contributed to the employment decline. Within the case of American factories, a lot of the manufacturing base has moved abroad that you may not have the ability to discover a factory that can create your product, and not all product categories are coated.
Control methods are sometimes carried out when creating medicine and manufacturing biological and therapeutic products, however, more and more they are thought of essential in the manufacture of sterile medicines. A number of structural shifts within the wooden products trade have contributed to the employment decline. Within the case of American factories, a lot of the manufacturing base has moved abroad that you may not have the ability to discover a factory that can create your product, and not all product categories are coated.