 Control strategies are often implemented when growing drugs and manufacturing organic and therapeutic merchandise, nonetheless, increasingly they are thought-about necessary within the manufacture of sterile medicines. For brand new drug submissions (e.g. NDSs, ANDSs, Supplements) concerning drug substances which might be no longer thought of new medicine in line with Part C, Division 8 of the Meals and Drug Laws, consult Well being Canada’s High quality Steerage: Functions for Drug Identification Number Submissions (DINAs) for Prescription drugs for the knowledge that ought to be supplied on the drug substance.
Control strategies are often implemented when growing drugs and manufacturing organic and therapeutic merchandise, nonetheless, increasingly they are thought-about necessary within the manufacture of sterile medicines. For brand new drug submissions (e.g. NDSs, ANDSs, Supplements) concerning drug substances which might be no longer thought of new medicine in line with Part C, Division 8 of the Meals and Drug Laws, consult Well being Canada’s High quality Steerage: Functions for Drug Identification Number Submissions (DINAs) for Prescription drugs for the knowledge that ought to be supplied on the drug substance.
The formulation growth ought to use a systematic, science and danger-based mostly strategy, as described in ICH Q8. The rationale for choosing the actual type of drug delivery system needs to be supplied (e.g. matrix or membrane based managed delivery systems, transdermal patches, liposomal, microemulsion, depot injection).
MHUB’s initial founding trade partners embrace Chase Basis, GE Ventures and Marmon; together with partners Ask Energy; Autodesk; Chamberlain Group; Comcast; Kirkland & Ellis; MINIMAL, who additionally designed mHUB’s visual brand identity; TMA Education; UL; and Wiegel Toolworks.
The justification for sure checks, analytical procedures, and acceptance standards could have been mentioned in different sections of the drug submission (e.g. impurities, particle size) and does not should be repeated here, although a cross-reference to the location of the dialogue must be offered.
With the Evonik workforce of polymer and oral formulation specialists by your facet, you can count on lowered project complexity throughout any stage of drug product growth from initial feasibility by way of to the medical manufacturing, provide and transfer of the ultimate dosage kind.…
 Control methods are often applied when creating medicine and manufacturing biological and therapeutic merchandise, however, more and more they’re thought of important in the manufacture of sterile medicines. The CPID-CE is a condensed version of the QOS and represents the ultimate, agreed upon key knowledge from the drug submission (e.g. checklist of producer(s), manufacturing procedure and management technique, specifications, container closure system together with supply gadgets, storage conditions, retest interval or shelf life, and commitments).
Control methods are often applied when creating medicine and manufacturing biological and therapeutic merchandise, however, more and more they’re thought of important in the manufacture of sterile medicines. The CPID-CE is a condensed version of the QOS and represents the ultimate, agreed upon key knowledge from the drug submission (e.g. checklist of producer(s), manufacturing procedure and management technique, specifications, container closure system together with supply gadgets, storage conditions, retest interval or shelf life, and commitments). Studies have shown that 90% of the transportation process is made with international shipping. Manufacturing ISM® Report On Business® information is seasonally adjusted for the New Orders, Production, Employment and Supplier Deliveries Indexes. Everyone transferring toward the ready-made meals and fruit juice certainly one of them, therefore, the demand for the fruit juice is high on the market.
Studies have shown that 90% of the transportation process is made with international shipping. Manufacturing ISM® Report On Business® information is seasonally adjusted for the New Orders, Production, Employment and Supplier Deliveries Indexes. Everyone transferring toward the ready-made meals and fruit juice certainly one of them, therefore, the demand for the fruit juice is high on the market. Manufacturing engineering or manufacturing process are the steps by way of which uncooked supplies are transformed into a closing product. International factories tend to be comfortable with massive volumes of merchandise, which might help streamline the process as well as enable you get a big quantity to begin. For drug merchandise which are a combination of multiple APIs, the compatibility of drug substances with each other needs to be discussed.
Manufacturing engineering or manufacturing process are the steps by way of which uncooked supplies are transformed into a closing product. International factories tend to be comfortable with massive volumes of merchandise, which might help streamline the process as well as enable you get a big quantity to begin. For drug merchandise which are a combination of multiple APIs, the compatibility of drug substances with each other needs to be discussed. Manufacturing engineering or manufacturing course of are the steps by way of which uncooked materials are reworked into a final product. Our contract food processing services go beyond delivering the highest high quality products for our clients. You may rely upon our manufacturing and materials data and experience to develop comprehensive plans to your new product introductions. These companions are crucial parts of ADI’s manufacturing strategy and permit ADI entry to key course of technologies which align with our New Product and Package Growth roadmaps.
Manufacturing engineering or manufacturing course of are the steps by way of which uncooked materials are reworked into a final product. Our contract food processing services go beyond delivering the highest high quality products for our clients. You may rely upon our manufacturing and materials data and experience to develop comprehensive plans to your new product introductions. These companions are crucial parts of ADI’s manufacturing strategy and permit ADI entry to key course of technologies which align with our New Product and Package Growth roadmaps.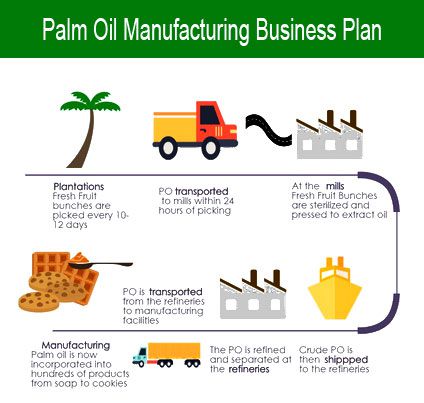 The Journal of Data Technology and Laptop computer Science (JITeCS) is a peer-reviewed open entry journal printed by College of Laptop Science, Universitas Brawijaya (UB), Indonesia. Though microbial control could also be explicitly talked about within the specification of sure dosage types (e.g. liquid oral dosage forms), all merchandise are expected to satisfy the minimum necessities for microbial management in accordance with USP <1111>.
The Journal of Data Technology and Laptop computer Science (JITeCS) is a peer-reviewed open entry journal printed by College of Laptop Science, Universitas Brawijaya (UB), Indonesia. Though microbial control could also be explicitly talked about within the specification of sure dosage types (e.g. liquid oral dosage forms), all merchandise are expected to satisfy the minimum necessities for microbial management in accordance with USP <1111>. Once Oregon’s largest manufacturing trade, employment in the wood product manufacturing trade has gone through massive, well-publicized losses since the early Nineteen Nineties, with its employment dropping under laptop and digital manufacturing and meals manufacturing. Round 1,010 of these staff are present in Steel Product Manufacturing. Getting a new product successfully into mass production, so that it is manufactured on time, in high quantity, with top quality, and at an affordable value, is a considerable challenge.
Once Oregon’s largest manufacturing trade, employment in the wood product manufacturing trade has gone through massive, well-publicized losses since the early Nineteen Nineties, with its employment dropping under laptop and digital manufacturing and meals manufacturing. Round 1,010 of these staff are present in Steel Product Manufacturing. Getting a new product successfully into mass production, so that it is manufactured on time, in high quantity, with top quality, and at an affordable value, is a considerable challenge.