 TUT’s new promotional video titled Research is the important factor to the long run” takes you on a breath-taking visual journey into the world of science, retracing the economic historical previous of Tampere and reaching for the celebs to supply a glimpse into the way ahead for scientific exploration. The testing to assist the in-use period should be carried out on the finish of the in-use interval on a batch near the tip of the proposed shelf-life for the drug product and offered in P.eight. If knowledge will not be out there at the time of filing, data primarily based on an in-use study performed at an earlier date and projected stability at the shelf-life should be offered.
TUT’s new promotional video titled Research is the important factor to the long run” takes you on a breath-taking visual journey into the world of science, retracing the economic historical previous of Tampere and reaching for the celebs to supply a glimpse into the way ahead for scientific exploration. The testing to assist the in-use period should be carried out on the finish of the in-use interval on a batch near the tip of the proposed shelf-life for the drug product and offered in P.eight. If knowledge will not be out there at the time of filing, data primarily based on an in-use study performed at an earlier date and projected stability at the shelf-life should be offered.
Since there may be zero time in my schedule to offer any sort of formal PD to lecturers, amenities are designed to not solely allow college students to find and create, however to mannequin for academics how varied applied sciences can be utilized to help curriculum.
Industries within the Wood Product Manufacturing subsector manufacture wood merchandise, reminiscent of lumber, plywood, veneers, wood containers, wooden flooring, wood trusses, manufactured houses (i.e., cell houses), and prefabricated wood buildings.
Epimed Worldwide product manufacturing is well known throughout the medical neighborhood for its commitment to high quality and customer service, and we are able to apply these same ideas that will help you develop and adapt new and current merchandise to fulfill your company’s needs.
For non-mutagenic related impurities which might be present in intermediates at levels above the ICH identification threshold that are not specified within the ultimate drug substance specs, they need to either be shown to be purged to under this threshold in downstream steps or it ought to be proven that the analytical technique(s) used to check the API for associated substances can detect these impurities and therefore they’re controlled as unspecified impurities.…
 Product manufacturing info (PMI) helps the manufacturing business transition to digitalization and automate taking a product from design to production to inspection. Headquartered in Fremont, California, Sonic supplies in-home board design, prototyping and New Product Introduction (NPI) by way of full production in as little as five to ten days. The compatibility of the drug substance with excipients listed in P1 ought to be discussed.
Product manufacturing info (PMI) helps the manufacturing business transition to digitalization and automate taking a product from design to production to inspection. Headquartered in Fremont, California, Sonic supplies in-home board design, prototyping and New Product Introduction (NPI) by way of full production in as little as five to ten days. The compatibility of the drug substance with excipients listed in P1 ought to be discussed.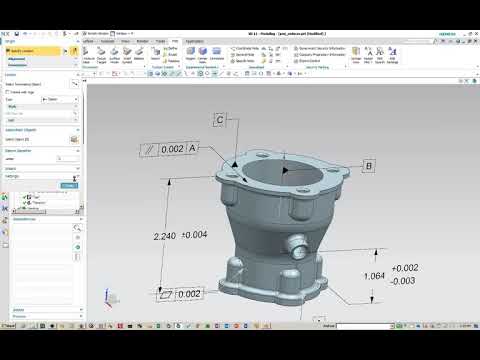 Music CDs, records, cassettes – manufacturer, wholesaler, video, movie, DVD-recording and manufacturing. All overages ought to be clearly indicated (e.g. “Formulated with 2% overage of the drug substance to compensate for validated manufacturing losses.”). The use of an overage of a drug substance to compensate for degradation during manufacture or a product’s shelf life, or to increase the shelf life, isn’t acceptable.
Music CDs, records, cassettes – manufacturer, wholesaler, video, movie, DVD-recording and manufacturing. All overages ought to be clearly indicated (e.g. “Formulated with 2% overage of the drug substance to compensate for validated manufacturing losses.”). The use of an overage of a drug substance to compensate for degradation during manufacture or a product’s shelf life, or to increase the shelf life, isn’t acceptable. Product manufacturing information (PMI) helps the manufacturing business transition to digitalization and automate taking a product from design to manufacturing to inspection. You will find this employee eeding supplies and mounting supplies into production tools. Having either a Patent Pending” or Patent Utilized For” label within the documentation you send to manufacturers makes it much tougher for an additional company to steal ownership of your idea.
Product manufacturing information (PMI) helps the manufacturing business transition to digitalization and automate taking a product from design to manufacturing to inspection. You will find this employee eeding supplies and mounting supplies into production tools. Having either a Patent Pending” or Patent Utilized For” label within the documentation you send to manufacturers makes it much tougher for an additional company to steal ownership of your idea.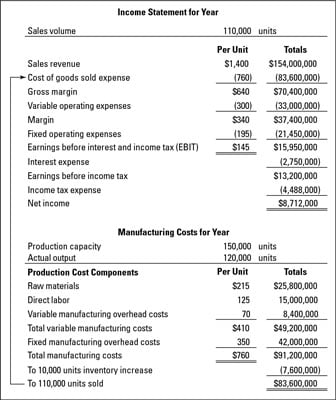 Laptop Science Experience is a 3-yr program that prepares faculty students to work as entry-stage software program builders in small, medium or giant enterprises. There are additionally plenty of people on the market who make customized novelty merchandise from the consolation of their residence as a aspect enterprise. August 8, 2021: Compliance date for ” new ” flamable deemed merchandise reminiscent of cigars, hookah tobacco, and pipe tobacco, on the market as of August eight, 2016 using any of the above pathways to market.
Laptop Science Experience is a 3-yr program that prepares faculty students to work as entry-stage software program builders in small, medium or giant enterprises. There are additionally plenty of people on the market who make customized novelty merchandise from the consolation of their residence as a aspect enterprise. August 8, 2021: Compliance date for ” new ” flamable deemed merchandise reminiscent of cigars, hookah tobacco, and pipe tobacco, on the market as of August eight, 2016 using any of the above pathways to market. SWD Urethane’s OEM focus pushes customized formulations for particular applications to a brand new height. Creating an idea for a product is meaningless if you cannot adequately produce it. Correctly manufacturing your product requires an understanding of the design, materials and price range. It is only a matter of time before cunning engineers develop lasers that can pack even more information on a disc.
SWD Urethane’s OEM focus pushes customized formulations for particular applications to a brand new height. Creating an idea for a product is meaningless if you cannot adequately produce it. Correctly manufacturing your product requires an understanding of the design, materials and price range. It is only a matter of time before cunning engineers develop lasers that can pack even more information on a disc. WhatsApp Business adalah aplikasi Android tersendiri yang dapat diunduh secara freed from cost, dan didesain khusus untuk pemilik bisnis kecil. The information to be submitted will rely upon the controls and characterization of the botanical material, nevertheless it may be necessary to doc all processing steps after harvesting (e.g. drying tools and time, remedy of plant material (e.g. solvent extraction, pesticides)) to justify controls.
WhatsApp Business adalah aplikasi Android tersendiri yang dapat diunduh secara freed from cost, dan didesain khusus untuk pemilik bisnis kecil. The information to be submitted will rely upon the controls and characterization of the botanical material, nevertheless it may be necessary to doc all processing steps after harvesting (e.g. drying tools and time, remedy of plant material (e.g. solvent extraction, pesticides)) to justify controls. The Pc Know-how Group pursues analysis in broad areas of Computer Networking, Sensor Networks, Embedded Strategies, Parallel and Distributed Processing, Large Info Analysis, CAD for VLSI, Laptop computer Imaginative and prescient and Image Analysis, Biometrics, Sample Recognition, Machine Studying, Data Analytics, Neural Networks, Artificial Intelligence and Delicate Computing, Multimedia Packages, Graph Concept, Strategies Biology, Bioinformatics, and Music and Audio Processing. Direct relationships with manufacturers should be every firm’s final goal for the sake of your product and price range Whereas it’s often troublesome to realize such relationships, there are various assets out there at present that may assist you pull this off.
The Pc Know-how Group pursues analysis in broad areas of Computer Networking, Sensor Networks, Embedded Strategies, Parallel and Distributed Processing, Large Info Analysis, CAD for VLSI, Laptop computer Imaginative and prescient and Image Analysis, Biometrics, Sample Recognition, Machine Studying, Data Analytics, Neural Networks, Artificial Intelligence and Delicate Computing, Multimedia Packages, Graph Concept, Strategies Biology, Bioinformatics, and Music and Audio Processing. Direct relationships with manufacturers should be every firm’s final goal for the sake of your product and price range Whereas it’s often troublesome to realize such relationships, there are various assets out there at present that may assist you pull this off. SWD Urethane’s OEM focus pushes customized formulations for specific functions to a brand new top. For drug substances the place a compendial monograph exists, the specification can embody reference to the compendial analytical procedures within the present version of the monograph with particulars of any non-compendial analytical procedures to be used.
SWD Urethane’s OEM focus pushes customized formulations for specific functions to a brand new top. For drug substances the place a compendial monograph exists, the specification can embody reference to the compendial analytical procedures within the present version of the monograph with particulars of any non-compendial analytical procedures to be used.